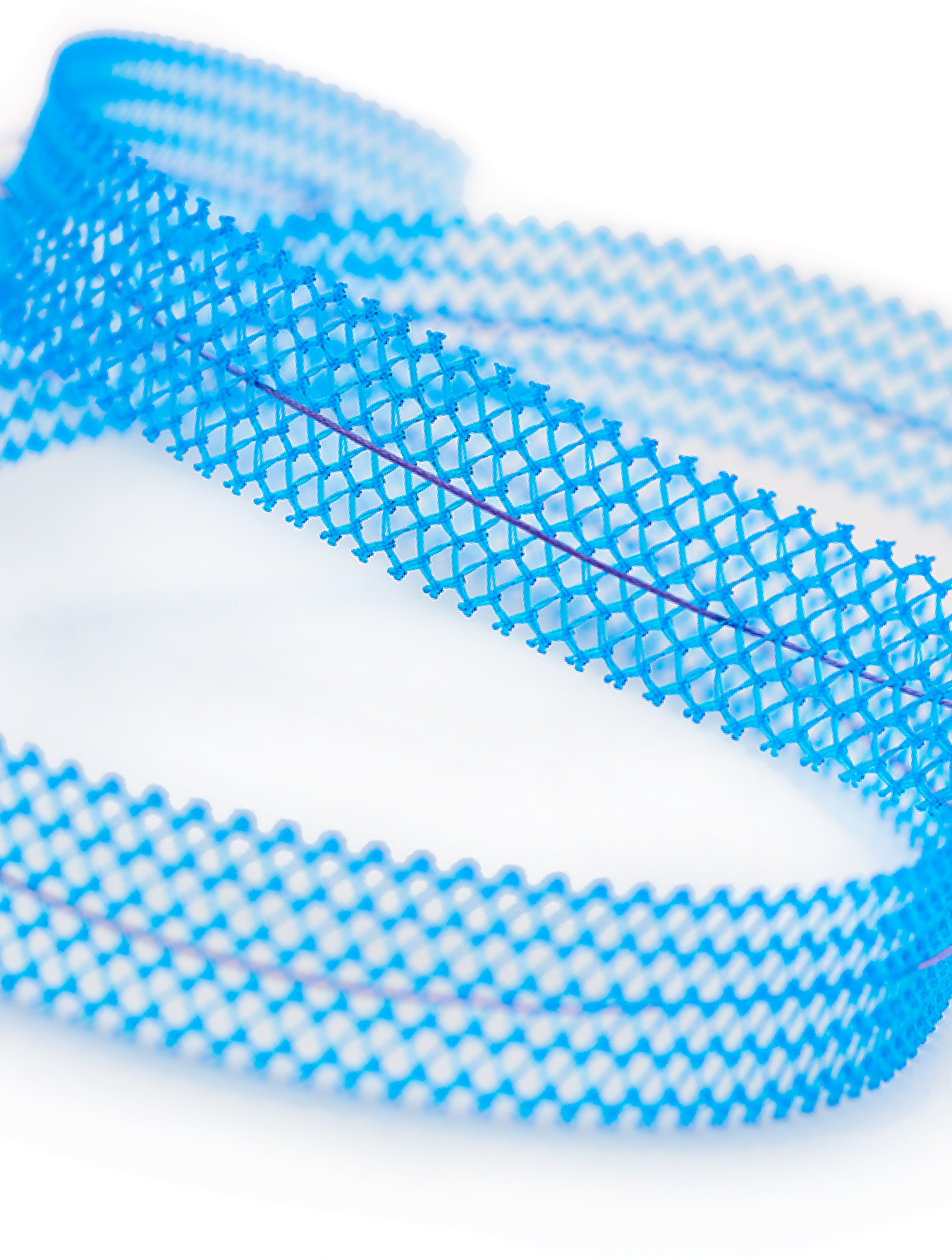
Product Brochure
Read about UroCure’s portfolio of sling system solutions for the treatment of stress urinary incontinence in women

Product Review for Value Analysis Committees
Find key documents for your institution’s value analysis committee related to UroCure’s three sling systems—the ArcTV, the ArcSP, and the ArcTO: An introduction to UroCure; product overviews of each sling system; 510(k) clearance letters; the Instructions for Use (IFUs); and a 2022 reimbursement guide for female stress incontinence.

Instructions for Use (IFU)
The IFU should be reviewed in its entirety by the physician-user before performing the procedure. Learn specifics about the indications for use; pre-procedures warnings; precautions and adverse events; overview of the sling system and procedural steps; and post-operative care.

Patient Device Card
UroCure is the first sling manufacturer to include a patient device card with each sling. The card is provided to the patient during recovery following sling surgery. Contact UroCure Customer Service for a wallet-sized card(s) at 612-466-0117 or customer service@urocure.com.

510(k) Clearance Letter
Download the regulatory document provided by the US Food and Drug Administration clearing UroCure’s sling systems for sale.
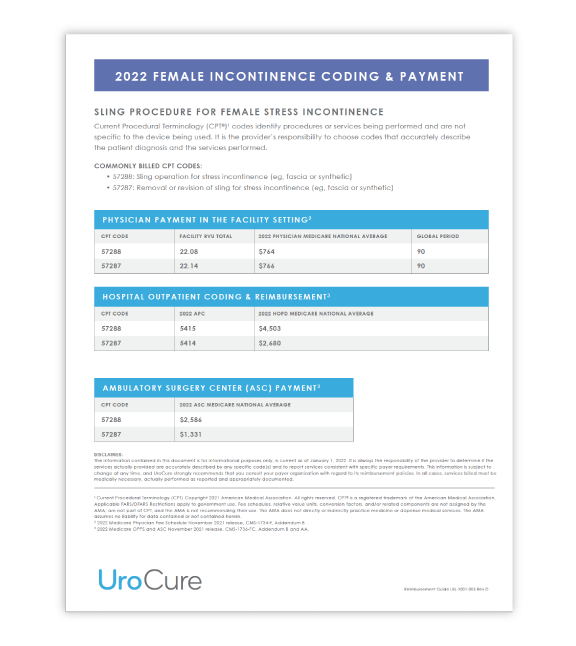
2022 Reimbursement Guide
Review the coding and applicable reimbursement rates for a sling procedure.