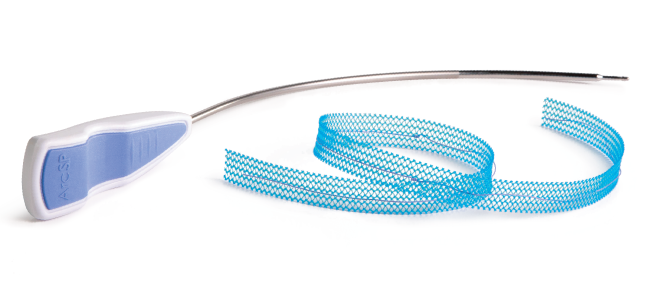
John Nealon and Dr. David Staskin form UroCure and secure intellectual property from Endo/AMS.
Read MoreUroCure raises $2.8M in Series A funding to finance the development of its first product, the ArcTV Sling System.
UroCure announces the ArcTV Patient Device Card, the industry’s first patient device card given to patients after their sling procedure. This provides the patient with the name, sling type, and device lot (tracking) number specific to her sling.
UroCure establishes the first Quality and Safety Oversight Committee (QSOC) in women’s health, comprised of leading academic experts in urogynecology and female urology.
UroCure announces partnering sponsorship with the American Urogynecologic Society (AUGS) for their ACQUIRE Registry to track patient outcomes with the ArcTV sling. Through their sponsorship, UroCure demonstrates its support for the society’s efforts to achieve quality patient outcomes and build transparency and trust among physicians, regulatory agencies, and industry.
Surgeons at Mt. Auburn Hospital—a Harvard Medical School teaching hospital–completes the first ArcTV Sling procedure.
Company announces completion of Series B funding of $1.8M, including $1M bridge financing.
UroCure and LiNA Medical announce a sales distribution and product development partnership that leverages the strengths of both companies.
As a result of AUGS’ decision to sunset its ACQUIRE registry, UroCure introduces a proactive text-based reporting system that will provide physicians with a seamless method of sharing feedback about their product experience with the company. UroCure is the first sling company to incorporate a means of gathering information about patient outcomes and post-surgical complications.
UroCure begins shipping every UroCure sling package with a QR code on the outside label: healthcare providers can scan this code, answer a brief set of questions on their experience with the UroCure sling in the operating room, and send their feedback directly to the company.
The FDA cleared UroCure’s ArcSP suprapubic and ArcTO transobturator sling systems, building out UroCure’s full range of surgical solutions for stress urinary incontinence.
UroCure and LiNA Medical USA ship the first ArcSP suprapubic slings. The ArcSP is one of two new products approved by the FDA in September of 2022.