A Portfolio of Leading-Edge Sling Solutions
Our sling systems are offered in three configurations to accommodate the surgeon’s preferred technique—bottom up [ArcTV], top-down [ArcSP], or outside-in [ArcTO]. Each of these handle-needle systems is noted for its ergonomic design, and incorporates UroCure’s best-in-class laser-cut sling with integrated stabilizing suture.
Click here to see what sets our sling apart.
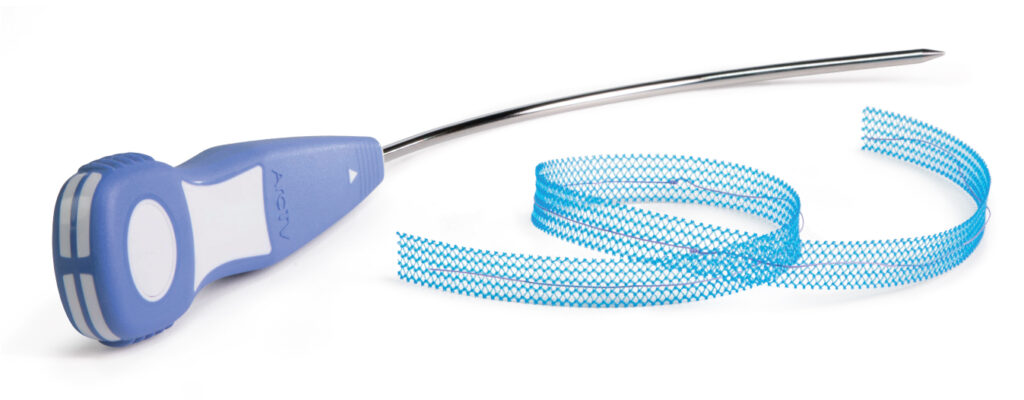
ArcTV Transvaginal Sling System
- Follows trajectory for transvaginal (bottom-up) needle passage
- Maintains sufficient rigidity and minimizes needle deflection during passage
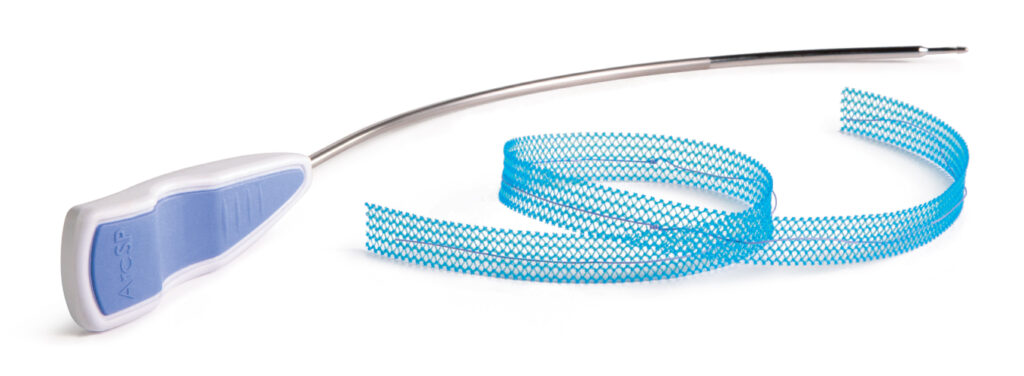
ArcSP Suprapubic Sling System
- Follows trajectory for suprapubic (top-down) needle passage
- Remains palpable and atraumatic during vaginal tunnel passage
ArcTO Transobturator Sling System
- Follows trajectory for transobturator (outside-in) needle passage
- With unique helical needle, allows single, continuous handle rotation during needle passage until vaginal incision is reached
