AUGS Fellows Hands-On Course
February 2020 Supporting physician training on the ArcTV sling is a cornerstone commitment for UroCure. UroCure sponsored and participated in…
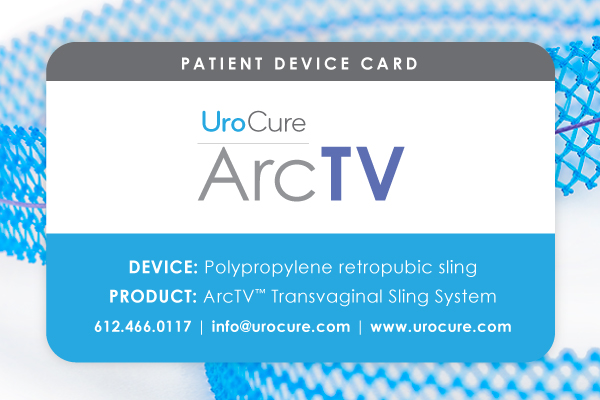
Patient Device Card
January 2020 UroCure reported on its first six months’ experience with the ArcTV Patient Device Card—the first patient device card…
First ArcTV Transvaginal Sling implanted at Mt. Auburn Hospital
July 2019 UroCure announces the first ArcTV Transvaginal Sling implanted at Mount Auburn Hospital, Cambridge, MA, affiliated with Harvard Medical…
UroCure Breaks New Ground: The ACQUIRE Registry
April 2019 UroCure announces a partnering sponsorship with the American Urogynecologic Society (AUGS) for their ACQUIRE Registry. The Registry will…
FDA Clearance
February 2019 Minneapolis On February 7, 2019, UroCure announced FDA 510(k) Clearance of its ArcTV® Transvaginal Sling System for the…